In the latest of thousands of pelvic mesh lawsuits in the U.S., Johnson & Johnson subsidiary Ethicon, Inc. was hit with a $41 Million verdict after a Philadelphia jury declared its mesh implants defective.
According to the lawsuit, Ethicon had not only negligently designed the products but also failed to warn they could erode through nearby organs and tissue. This case left a woman with permanent injuries after an implant sawed into her vagina.
This marks Ethicon’s eighth pelvic mesh lawsuit in Philadelphia since 2015 and the sixth to award multimillion-dollar damages against the company, including a $35 Million verdict last year. The grand total? More than $145 Million.
What Went Wrong in This Pelvic Mesh Lawsuit?
In 2007, the plaintiff received implants of 3 of Ethicon’s pelvic mesh devices (Gynemesh, Prolift, and TVT-O) to treat stress incontinence and organ prolapse. But mere weeks passed before she began suffering adverse events, according to her attorney. A portion of 1 device had begun to erode into her vagina, the lawsuit alleged, causing infections, inflammation, bleeding, and pain, among other symptoms.
Even after multiple revision surgeries – in themselves, risky procedures that threaten nearby nerves and organs – the plaintiff continued to experience complications. In total, according to the lawsuit, she underwent 9 revision surgeries to fix 14 mesh erosions.
The plaintiff’s counsel urged jurors to hold Ethicon accountable for failing to test its products properly before putting them on the market. Unanimously, the jury agreed that Ethicon misrepresented the products’ safety and efficacy. The plaintiff won $25 Million in punitive damages, $15 Million in compensation, and $1 Million for her husband’s loss of consortium.
The Dangers of Pelvic Mesh
As in the Philadelphia woman’s case, pelvic mesh (also known as transvaginal mesh) is widely used worldwide to treat stress incontinence, a common condition that can cause leaking from the bladder in women after childbirth and menopause. Likewise, up to half of new mothers suffer from pelvic organ prolapse, which occurs when the bladder, rectum, or uterus “sags” from damage to the pelvic floor muscles during childbirth.
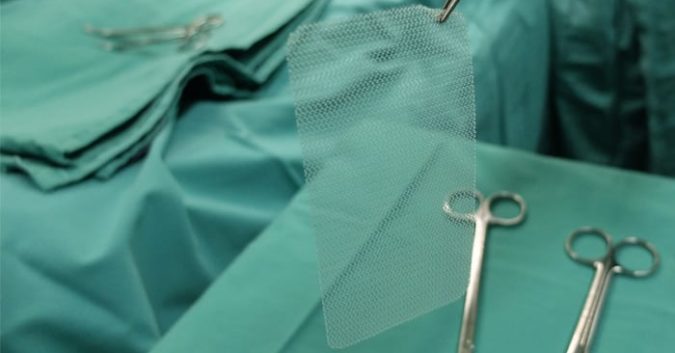
The mesh, a net-like implant that supports and repairs any damaged pelvic organs and tissue, has a low complication rate in general. But when things go wrong, the complications can be severe. Women have reported:
- Mesh exposure and erosion (when the mesh pokes through the vaginal wall)
- Vaginal scarring
- Fistula formation (when an abnormal passage forms between the vagina and another organ)
- Painful sexual intercourse
- Pelvic, back, and leg pain
Some complications occur years after surgery and can be difficult to treat. Indeed, despite limited research, UK data shows that 1 in 15 women require surgery to have the mesh removed. And though relatively rare, serious complications affect high numbers of women given the thousands who undergo mesh surgeries. Each year in the U.S., 560,000 women collectively undergo surgery to treat incontinence or prolapse, a third of those surgeries using mesh.
As quickly as pelvic mesh has grown more popular over the years, a growing body of evidence has shown it’s less effective for treating organ prolapse. Meanwhile, more and more women have come forward to report complications. Finally, in 2012, the U.S. Food and Drug Administration (FDA) reclassified pelvic mesh as a high-risk device.
100,000 Lawsuits (and Counting)
Despite the dangers, Ethicon hasn’t changed its defense: that the risks of mesh devices were well-known to doctors and the devices were properly designed. The company went as far to argue in the latest case that despite complications, the products had at least done their job in treating the woman’s incontinence.
“Pelvic organ prolapse and stress urinary incontinence are serious and debilitating conditions with limited treatment options,” the spokesperson said in a statement saying Ethicon plans to appeal the jury’s decision. “While we empathize with those who have experienced complications, many women with pelvic mesh see an improvement in their day to day lives.”
Yet a sizeable portion of women do not. More than 100,000 pelvic mesh lawsuits have been filed in the U.S. – Ethicon facing the majority of them – by women who say the problem deserves more attention.
Should you be worried if you or a loved one underwent surgery with Ethicon products? Experts say, perhaps not. Statistically, you are unlikely to suffer adverse events. But if you do, you are encouraged to see a doctor and within your rights to hold Johnson & Johnson accountable as thousands of other women have. Learn how to do that here.