What Is the Depo Shot Lawsuit in 2025?
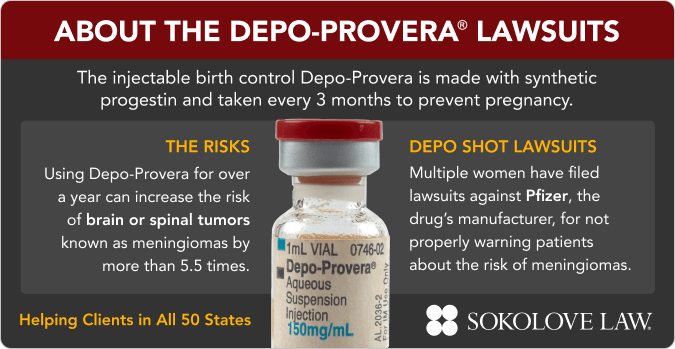
Depo-Provera lawsuits against Pfizer Inc. claim the company didn’t warn patients about the risk of brain tumors associated with this medication. These lawsuits aim to hold Pfizer accountable for their negligence and seek compensation for women who developed meningiomas after using Depo-Provera.
According to a March 2024 study in the BMJ, women who use Depo shots for at least a year are 5 times more likely to develop meningioma brain tumors. These tumors can cause serious neurological side effects — but were not listed on the injection's warning label.
About Depo Shot Lawsuits
- The first Depo-Provera meningioma lawsuit was filed in October 2024 by a woman who used the shots between 2005 and 2021.
- Multiple lawsuits have since been filed across the U.S., alleging that Pfizer knew or should’ve known about the link between this medication and brain tumors.
- Pfizer still hasn’t updated the Depo shot warning label in the U.S. to include the risk of brain tumors.
- As of September 2025, over 800 of these claims have been consolidated in a multidistrict litigation (MDL), which is similar to a Depo-Provera class action lawsuit.
- Previously, Pfizer was ordered to pay more than $2 Million in Depo shot settlements for not properly warning patients about the risk of bone mineral density loss associated with this contraceptive.
Around 24.5% of all sexually active women in the U.S. have used Depo-Provera at some point, putting a significant number of Americans at risk. Those affected may be able to file a Depo-Provera cancer lawsuit against Pfizer.
"I finally reached a law firm that truly understands the gravity of damage done by the Depo manufacturer."
– Syble, Depo-Provera Victim in Georgia
At Sokolove Law, we’ve helped families in all 50 states, securing more than $1.6 Billion from dangerous drug and medical device cases nationwide. Let our Depo shot lawsuit lawyers fight for you.
Get the Help You Deserve
Our experienced attorneys may be able to file a Depo-Provera lawsuit and fight for compensation on your behalf. See if you qualify now.
The Troubling History of Depo-Provera Injections
The U.S. Food & Drug Administration initially approved Depo-Provera in 1959 for managing menstruation. The drug was later approved as a contraceptive in 1974, but the FDA reversed this decision in 1978 due to concerns it may cause cancer.
By 1992, Depo-Provera was back on the market. Yet just 12 years later, the FDA issued a black box warning for the contraceptive over a significant loss of bone mineral density in long-term users.
A black box warning is the most serious warning the FDA can issue for a prescription medication. It alerts patients and doctors to dangerous or potentially life-threatening risks associated with a drug’s use.
This side effect led to a Depo shot class action lawsuit that resulted in a settlement worth over $2 Million in 2021.
Years ago, Canada and the European Union added a warning about the potential link between Depo-Provera and meningiomas to the drug’s label. However, users in the U.S. were left unaware of this risk.
Who Qualifies for a Depo Shot Lawsuit?
Eligibility for the Depo shot lawsuit is determined by your use of the birth control injections, the duration of use, the injury you suffered as a result, and any treatments undergone.
You may qualify to file a Depo shot lawsuit if you or a loved one:
- Used Depo-Provera, Depo-SubQ Provera 104, or authorized generics for over a year
- Developed a type of brain or spine tumor known as a meningioma
- Underwent surgery or radiation or have these treatments scheduled for the future
Filing a Depo-Provera lawsuit may result in compensation that can help families pay for treatment and make up for any lost wages. However, you only have a limited amount of time, so be sure to act fast.
Call (800) 995-1212 now to see if you may qualify. It costs nothing to speak with us.
Statute of Limitations on Depo-Provera Lawsuits
There’s only a limited amount of time to file a Depo-Provera brain tumor lawsuit due to state laws called statutes of limitations.
In most states, you have around 2-3 years after your diagnosis to file a Depo shot lawsuit against Pfizer.
Once the deadline in your case passes, you won’t be able to pursue compensation for meningiomas again.
Depo-Provera Shot Lawsuit Updates 2024-2025
Depo-Provera was first used as a form of birth control in 1974. Since then, millions of women have received the Depo contraceptive shot — and some may have developed brain tumors as a result.
Now, victims and their families are seeking justice by filing lawsuits against the company responsible for their condition. Find out more about the latest updates on the Depo-Provera shot lawsuit.
1. Pfizer Claims the FDA Rejected the Meningioma Warning Label
Latest Depo-Provera Lawsuit Update | September 2025
Pfizer has filed a motion for summary judgment, which is a legal request asking the court to dismiss certain Depo-Provera claims before they proceed to trial.
The company argues that since the U.S. Food & Drug Administration (FDA) didn't require them to warn about meningiomas, federal law should override state-based failure to warn claims. A hearing on the issue is scheduled for later this month.
2. New Study Strengthens Depo-Provera Claims
Depo-Provera Litigation Update | August 2025
A recent study found that women who use Depo-Provera for over a year are 3.5 times more likely to develop meningiomas compared to those taking oral contraceptives that contain ethinylestradiol-levonorgestrel.
Published in Expert Opinion on Drug Safety, these findings further highlight the association between long-term Depo-Provera use and increased meningioma risk.
3. Discovery Ongoing in the Birth Control Shot Lawsuit
Depo Shot Lawsuit Update | July 2025
Pfizer has turned over more than 8,000 privileged documents as part of the discovery process, which is when both sides exchange information that may be used as evidence in court.
These records could provide insight into what the company knew about potential risks associated with the birth control shot and how they communicated those risks to the public.
The judge overseeing the Depo-Provera litigation noted that the process has been moving forward efficiently so far, largely due to the cooperation between attorneys on both sides.
4. Depo-Shot Lawsuits Are Now Under Review
Depo-Provera Lawsuit News | June 2025
A third party has begun reviewing all complaints filed in the Depo-Provera MDL to ensure they meet the court’s requirements before moving forward in the legal process.
If a complaint is missing critical information, plaintiffs will be notified and given 2 business days to make corrections in order to prevent their cases from being delayed or dismissed.
5. Court Establishes Review Process for Depo Shot Claims
Birth Control Shot Lawsuit Update | May 2025
The court has approved a process to identify and correct potential issues in plaintiff complaints. All pending cases will be reviewed to ensure they include proof of a qualifying diagnosis following the use of Depo-Provera or an authorized generic.
For the purpose of this litigation, qualifying diagnoses include various types of meningiomas. The approved Depo-Provera formulations include Depo-Provera, Depo-SubQ Provera 104, and other brand or generic versions containing medroxyprogesterone acetate.
6. Plaintiffs Must Submit Proof of Use in Depo-Provera Lawsuit
Depo-Provera Litigation Update | April 2025
Legal teams in the Depo-Provera MDL have until July 2025 to submit a Plaintiff Proof of Use/Injury Questionnaire verifying their client’s use of the drug and subsequent meningioma diagnosis.
Documents to prove this information may include:
- A prescription
- Medical records
- Pharmacy records
The order applies to all cases filed before it was issued, as well as any future claims transferred to the MDL. Plaintiffs will have 120 days from the order or from the date their case is transferred to complete and return the questionnaire.
7. First Depo-Provera Cases Selected for Trial
Update on Depo-Provera Lawsuits | March 2025
Five Depo-Provera cases have been selected from the MDL to serve as the first lawsuits to go before a jury. These initial trials, called bellwether cases, are designed to test the strength of the claims.
The selected Depo-Provera lawsuits include:
- Blonski v. Pfizer Inc. et al.: Allison Blonski began using Depo-Provera in 2002. After experiencing severe headaches and arm twitching, doctors discovered a meningioma. Nearly a decade later, a second meningioma was diagnosed.
- Schmidt v. Pfizer Inc. et al.: Kristina Schmidt received approximately 64 Depo-Provera injections. Over time, she developed headaches, dizziness, and vertigo. She was diagnosed with an intracranial meningioma and underwent surgery..
- Toney v. Pfizer Inc. et al.: Donna Toney received her first Depo-Provera injection in 1997. In 2011, she began experiencing vertigo, dizziness, and hearing loss. An MRI revealed a meningioma, which was surgically removed.
- Valera-Arceo v. Pfizer Inc. et al.: Rachel Valera-Arceo used Depo-Provera for 7 years. After developing headaches, vision problems, and jaw pain, she was diagnosed with a meningioma and underwent brain surgery and radiation.
- Wilson v. Pfizer Inc. et al.: Alicia Wilson used Depo-Provera from 1998 until 2019. After suffering a stroke, doctors discovered a Grade 1 meningioma.
Bellwether trials play a critical role in mass tort litigation. The outcomes can influence potential settlements and may provide insight into how a jury will respond to arguments involving brain tumors and the Depo shot.
8. Depo-Provera MDL Established in Florida
Depo Shot Lawsuit Update | February 2025
Depo-Provera brain tumor lawsuits have officially been consolidated into an MDL in the Northern District of Florida, where multiple Depo shot claims were already pending.
Judge M. Casey Rodgers, who previously oversaw the largest mass tort in the nation, will preside over the litigation. With a judge assigned, discovery, pretrial motions, and potential bellwether trials will be coordinated in a single court.
9. Federal Judges to Hear Case for Consolidation
Birth Control Shot Lawsuit News | January 2025
This month, a panel of federal judges will hear arguments on whether Depo-Provera lawsuits should be consolidated into an MDL and, if so, where the MDL should be established.
A group of plaintiffs is pushing for consolidation in the Northern District of California, while Pfizer is seeking centralization in the Southern District of New York, which is closer to the company’s headquarters.
10. Motion Filed to Form Depo-Provera MDL
Depo-Provera Shot Lawsuit Update | December 2024
A motion was filed with the Judicial Panel on Multidistrict Litigation to consolidate over a dozen Depo-Provera lawsuits in a multidistrict litigation (MDL), which is similar to a class action lawsuit.
Consolidation is often requested when multiple lawsuits involve similar claims about the same drug or injury. In the case of Depo-Provera, plaintiffs allege the birth control shot led to the development of meningiomas, a type of brain or spinal tumor.
If approved, Depo-Provera lawsuits would be transferred to a single court, where they would be overseen by the same judge. The goal of consolidation is to streamline proceedings and make the legal process more efficient.
11. Additional Victims File Depo-Provera Lawsuits
Depo-Provera Litigation Update | November 2024
Multiple Depo-Provera lawsuits have been filed in state courts across the country. These lawsuits aim to hold Pfizer accountable for failing to warn patients about the risk of meningiomas.
Given the number of women who may have been impacted by this medication, a Depo-Provera class action lawsuit or multidistrict litigation may form in the future.
12. First Depo-Provera Lawsuit Filed
Depo-Provera Lawsuit News | October 2024
The first Depo-Provera lawsuit was filed by a California woman who was diagnosed with a meningioma at 37 years old. She received around 64 Depo-Provera injections between 2005 and 2021.
In 2022, after experiencing headaches, dizziness, and vertigo, she underwent an MRI. The victim later had surgery to remove a brain tumor.
13. Depo-Provera Brain Tumor Patient Speaks Out
Depo Shot Lawsuit Update | July 2024
Lucy Woodward, a nurse and mother of 3, publicly shared her story after undergoing meningioma surgery. She had severe headaches and trouble understanding others following her use of Depo-Provera, which led to the discovery of a golf ball-sized brain tumor.
Her story has drawn attention to the potential risks of Depo-Provera and the need for better warnings to protect women from dangerous side effects.
14. Pfizer Acknowledges Depo Shot Brain Tumor Study
Depo-Provera Litigation Update | April 2024
Pfizer, the manufacturer of Depo-Provera, acknowledged the findings of the BMJ study and stated they would work with regulatory agencies to update product labels.
However, despite warning labels for Depo shots in Canada already mentioning the risk of meningiomas, Pfizer has yet to update labels for products sold in the U.S.
15. Study Connects Depo-Provera to an Increased Risk of Meningiomas
Depo-Provera News | March 2024
A study published in the BMJ reported that patients receiving medroxyprogesterone acetate injections — the active ingredient in Depo-Provera — may be at an increased risk of developing meningioma brain tumors.
Women who take Depo-Provera for over 1 year may be 5 times more likely to develop a meningioma, according to the study.
16. Researchers Find Link Between Tumors and Progesterone
Update on Depo-Provera | June 2023
The growth of spinal meningiomas is linked to the hormone progesterone, according to Neuro-Oncology Advances.
The Depo shot contains a synthetic version of progesterone called medroxyprogesterone acetate, which mimics the effects of this hormone.
Our team is committed to staying up-to-date on this litigation and providing readers with the latest news on the Depo-Provera lawsuit. If you or a loved one has been diagnosed with a meningioma, contact us now.
How to File a Depo-Provera Lawsuit
To file a Depo-Provera lawsuit, contact an attorney with experience in drug injury claims to evaluate your case, determine your eligibility, and build your case. Companies like Pfizer usually have legal teams of their own, which makes it important for you to as well.
At Sokolove Law, we strive to make filing a Depo-Provera brain tumor lawsuit as easy and stress-free as possible by handling the process on your behalf. This way, you can focus on your recovery and family, while we handle the legal work.
Filing a Depo-Provera lawsuit generally involves your legal team:
- Determining your eligibility to file a lawsuit during a free case review
- Gathering evidence like medical records and expert testimony to establish a link between your tumor and Depo-Provera use
- Filing your Depo shot claim before any deadlines
- Negotiating a Depo-Provera lawsuit settlement on your behalf
If a settlement isn’t reached, we’re prepared to present your case in court and pursue compensation from a trial verdict instead. We’ll fight hard to hold Pfizer accountable and get you justice.
Billions Recovered Nationwide
At Sokolove Law, we’ve recovered over $1.6 Billion for thousands of injured clients across the country. Let us get you the money you deserve.
Depo-Provera Lawsuit Settlements 2025
If you were diagnosed with a meningioma tumor and used Depo-Provera at least 4 times, you may be eligible for compensation from a Depo shot settlement.
In legal cases, pharmaceutical manufacturers often agree to out-of-court settlements that pay out a specific amount to those harmed by their products.
Depo-Provera lawsuit settlements may provide:
- Quicker access to compensation: Settlements often avoid the need for a full trial and can allow families to get the money they need faster.
- Greater certainty: Patients are guaranteed to receive the amount agreed upon by both parties.
- Security: Depo shot settlements can’t be reduced or appealed in the future like a trial verdict can.
In 2021, a Depo class action lawsuit resulted in a $2 Million settlement over Pfizer not including bone density loss on their warning labels in Canada.
A Depo-Provera lawsuit settlement can provide families with peace of mind and financial relief for those facing a brain tumor caused by this birth control medication.
Determining Depo Shot Settlement Amounts
It’s difficult to predict how much Depo shot lawsuit settlements may be worth because potential payouts will vary based on the unique circumstances of each case.
However, one study found that the average settlement in lawsuits involving meningiomas was over $800,000, while the average trial verdict awarded more than $3 Million.
Factors that may impact Depo shot settlement amounts include:
- The severity of your condition: Settlement amounts often consider the size of the tumor, if it has spread, and how it affects your quality of life since symptoms like vision loss can be debilitating.
- Related medical bills: Both past and future costs for treatments, surgeries, and other procedures related to the tumor will impact the amount of compensation you may receive.
- Any lost wages: If the tumor has caused you to miss work or limited your ability to earn a living, this lost income can be factored into the settlement.
- Whether the case settles or goes to trial: A trial verdict may result in a larger award, but there’s no guarantee of compensation.
At Sokolove Law, we’ll fight hard to get you everything you’re entitled to. Call (800) 995-1212 now to see if we can fight for you.
Choosing a Depo-Provera Lawsuit Lawyer
When choosing a Depo-Provera lawyer, it’s important to find someone who has the knowledge and resources to secure the compensation you deserve.
At Sokolove Law, we’re prepared to fight hard on your behalf and seek justice from the company responsible for your condition.
Find out how our Depo-Provera lawsuit lawyers stand out from the rest:
- Decades of Experience: For over 45 years, our team has stood up to negligent corporations that have harmed innocent consumers.
- Nationwide Reach: With offices and attorneys across the country, we can help families located anywhere in the United States.
- No Upfront Costs: Our Depo-Provera attorneys don’t charge any upfront costs or hourly fees.
- Track Record of Success: We’ve recovered over $10 Billion total for those injured through no fault of their own.
We understand the complexities of pharmaceutical litigation and can help level the playing field between you and powerful drug companies. Let us put our decades of experience to work for you.

"Our mission at the firm is simple. It’s to provide everyone, regardless of education, background, or social status, with equal access to the legal system.”
– Jim Sokolove, Founder (Retired 2013)
Depo-Provera and Meningioma Risks
Depo-Provera (medroxyprogesterone acetate) is a progestin-based birth control injection given every 3 months. However, when used for over a year, it may cause patients to develop tumors known as meningiomas.
A March 2024 study that examined the cases of over 18,000 women who had surgery for intracranial meningiomas highlighted concerns about the potential connection between long-term Depo-Provera use and these tumors.
About Depo-Provera and Meningioma Risks
- Women were found to be over 5 times more likely to suffer from meningiomas after using Depo-Provera for over a year, according to the BMJ.
- Exposure to medroxyprogesterone acetate, the active hormone in Depo shots, may increase the risk of these tumors.
- Meningiomas often have receptors for hormones like progesterone. Prolonged use of Depo shots increases exposure to a synthetic form of progesterone, which can cause tumor growth.
Meningiomas grow in the protective layers of tissue surrounding the brain and spinal cord. Because of where these tumors form, they may put pressure on the brain and cause patients to experience neurological symptoms.
Around 10-15% of meningiomas are cancerous, which can cause additional complications. The cost of treating brain tumors like meningiomas may be upwards of $700,000 before insurance.
Depo-Provera Brain Tumor Symptoms
Meningiomas, the type of brain tumor that can be caused by Depo-Provera, may not be detected for years due to their slow growth.
The threat posed by meningiomas depends on their size, location, and growth rate. As the tumors increase in size, they can push on different parts of the brain and cause various neurological issues.
Depo-Provera brain tumor symptoms may include:
- Blurred vision
- Confusion
- Double vision
- Hearing loss
- Loss of smell
- Memory loss
- Seizures
- Trouble speaking
- Vision loss
- Weakness in the arms or legs
By filing a Depo-Provera lawsuit, you may be able to get help paying for medical bills and other costs associated with your treatment.
Get the Help You Deserve
Our experienced attorneys may be able to file a Depo-Provera lawsuit and fight for compensation on your behalf. See if you qualify now.
Depo-Provera Meningioma Treatments
While small, slow-growing meningiomas may not need treatment right away, tumors that grow large enough to press on the brain and cause symptoms may need to be removed or treated.
Treatments for meningiomas from Depo-Provera may include:
- Surgery: A surgeon will attempt to remove the entire meningioma. If the tumor's location makes complete removal difficult, they’ll aim to remove as much as possible.
- Radiation: When tumor cells remain after surgery or surgery isn’t an option, doctors will use radiation to try and destroy the remaining meningioma cells.
- Chemotherapy: Although rare for meningiomas, chemotherapy may be used if the tumor doesn’t respond well to surgery or radiation.
Your doctor’s recommended treatment approach may depend on many factors, including the tumor’s location, size, and grade.
Meningiomas are classified into 3 grades: Grade 1 grows slowly and is the most common, Grade 2 is more likely to invade the brain and has a higher chance of growing back, and Grade 3 is the most aggressive and often cancerous.
The cost of treating a brain tumor can add up quickly, with medical expenses sometimes rising as high as $700,000 before insurance.
If you have a brain tumor related to using the Depo shot, you may be eligible for compensation from a Depo-Provera meningioma lawsuit.
Find a Depo Shot Lawsuit Lawyer Near You
At Sokolove Law, we have over 45 years of experience holding powerful pharmaceutical companies accountable. Our Depo shot lawyers can help families in all 50 states seek justice for meningiomas.
We’ve secured more than $1.6 Billion for patients harmed by dangerous drugs and medical devices.
There are no upfront costs or hourly fees to work with our team. Our Depo-Provera attorneys operate on a contingency-fee basis, which means we only get paid if you do.
Call (800) 995-1212 now or fill out our contact form to get started with a free, no-obligation case review.
Depo-Provera Brain Tumor Lawsuit FAQs
What is the Depo-Provera shot lawsuit about?
Depo-Provera shot lawsuits allege that Pfizer, the drug’s manufacturer, knew or should have known their product may increase the risk of meningioma brain tumors. However, many patients feel they weren’t properly warned about this.
As of September 2025, at least 800 Depo shot lawsuits have been consolidated into a multidistrict litigation (MDL) in the Northern District of Florida.
If you or a loved one developed a brain tumor after using Depo-Provera, contact us now. You may be able to pursue compensation from a Depo-Provera lawsuit.
Can you sue for a Depo shot?
Yes. Women who received multiple Depo-Provera shots and were later diagnosed with a meningioma brain tumor may be able to sue Pfizer for their injuries.
Depo shot meningioma lawsuits may allow patients to seek justice and compensation for the pain they’ve experienced.
How do I file a lawsuit against Depo-Provera shots?
To file a lawsuit against Depo-Provera shots, reach out to an experienced attorney who can guide you through the legal process and pursue compensation on your behalf.
By working with our Depo-Provera attorneys, you can feel confident knowing we have the resources and skills needed to level the playing field between you and powerful companies like Pfizer.
Call (800) 995-1212 now to get started on your Depo shot claim.
How much does hiring a Depo-Provera lawsuit lawyer cost?
At Sokolove Law, there are no upfront costs or hourly fees to work with our Depo-Provera lawsuit lawyers.
We only get paid if we secure compensation for you, so there’s no financial risk to taking legal action.
Is there a Depo-Provera class action lawsuit in 2025?
As of September 2025, over 800 claims have been consolidated into a multidistrict litigation (MDL), which is similar to a Depo-Provera class action lawsuit.
These lawsuits are filed against Pfizer, the maker of Depo-Provera, by women claiming the drug caused them to develop brain tumors.
Find out if you may qualify for a Depo-Provera lawsuit during a free case review.
How long do I have to file a Depo-Provera cancer lawsuit?
In most states, you typically have 2-3 years after your diagnosis to file a Depo-Provera cancer lawsuit.
This deadline varies by state, so it’s important to talk with an experienced attorney and find out more about your options.
What does Depo-Provera birth control do to the brain?
Depo-Provera may increase the risk of brain tumors called meningiomas, which can cause neurological issues like vision loss and seizures.
If you or a loved one developed a brain tumor after using Depo-Provera, you may be eligible for compensation from a lawsuit against the drug’s manufacturer.
Call (800) 995-1212 now to see if our Depo-Provera lawyers can fight for you.
Who makes Depo-Provera birth control?
Depo-Provera is made by Pfizer Inc., a major pharmaceutical company known for creating a wide range of medications and vaccines.