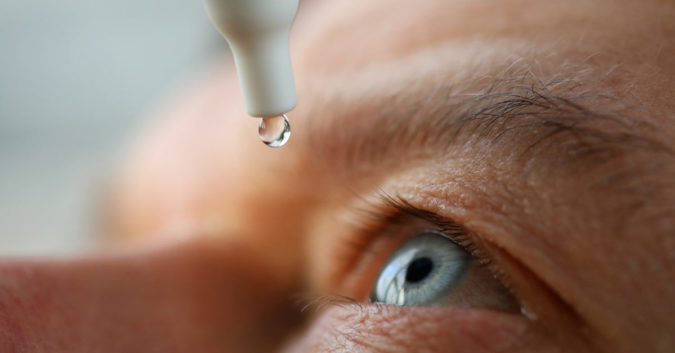
On February 2, 2023, the U.S. Food and Drug Administration (FDA) confirmed EzriCare® Artificial Tears are under a voluntary recall after the product was linked to dozens of reports of serious eye infections and one death.
More than 50 people across 12 states — California, Colorado, Connecticut, Florida, New Jersey, New Mexico, New York, Nevada, Texas, Utah, Washington, and Wisconsin — developed a rare bacterial infection after using EzriCare eye drops. Because EzriCare is sold at common drug stores across the nation, including Walmart, Target, and CVS, and one of the top products on online retailers, additional consumers could be at risk of infection.
At this time, the Centers for Disease Control and Prevention (CDC) and FDA are continuing to investigate both opened and unopened bottles of EzriCare Artificial Tears manufactured by Global Pharma Healthcare. Delsam Pharma, another brand that distributes Global Pharma Healthcare eye drops, is also included in the voluntary recall.
EzriCare and Global Pharma Healthcare have halted distribution and sale of the product until additional updates are provided from the CDC and FDA.
Contaminated Products and Drug-Resistant Infections
The CDC began investigating the product when dozens of patients across the U.S. began developing serious eye infections. After several tests, doctors found the infections were caused by a rare bacteria known as Pseudomonas aeruginosa.
These infections are incredibly dangerous because of bacteria’s unique abilities to resist antibiotics. Eye infections from this strain of bacteria can cause keratitis and endophthalmitis, which impact the cornea and eye cavity and lead to vision impairment or blindness.
Additionally, since the bacteria is so drug-resistant it continues to spread throughout the body, leading to respiratory infection such as pneumonia, urinary tract infection, and sepsis. Of the 55 known cases so far, one patient has passed away from infection that spread throughout the body.
The main link between all patients was use of the EzriCare multidose eye drops. Because EzriCare is a preservative-free product that can be used multiple times, the risk of bacterial overgrowth after opening and use is high, especially if contaminated during manufacturing.
The CDC and the FDA are still studying whether the bottles were contaminated during manufacturing or if the bacterial overgrowth occurred after the bottles were opened.
Next Steps for Consumers
Any patients using EzriCare eye drops are recommended to stop using the product immediately and monitor for any emerging infection symptoms.
Symptoms of an eye infection may include:
- Blurred vision
- Discomfort
- Inflammation
- Pain and sensitivity
If you have used EzriCare eye drops and developed an infection or other health issues, contact your doctor immediately. You may also be eligible to file an EzriCare lawsuit that can help you pay for resulting medical treatment.
For over 45 years, Sokolove Law has held pharmaceutical manufacturers accountable for their dangerous products.